Rate-determining step
The rate-determining step (RDS) is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In similar manner, the rate of reaction depends on the rate of the slowest step. However it is not clear what 'slowest step' means. For example does it refer to the step with the smallest rate constant; if the reaction is reversible, does it refer to the ratio of the forward and reverse rate constants or does it simply refer to the step that has the smallest flux? If the latter, then at steady state all steps carry the same flux and therefore there is no slowest step. In metabolic pathways the rate limiting step is better defined and a measure, the flux control coefficient is given to a step to signify how rate-limiting the step is. Theory and experiment also suggests that there is no single rate limiting step but a range of rate limitingness across the entire reaction network.
For example, the reaction NO2(g) + CO(g) → NO(g) + CO2(g) can be thought of as occurring in two elementary steps:
- NO2 + NO2 → NO + NO3 (slow step)
- NO3 + CO → NO2 + CO2 (fast step)
As the second step consumes the NO3 produced in the slow first step, it is limited by the rate of the first step. For this reason, the rate-determining step is reflected in the rate equation of a reaction.
Another example of a rate-determining step is the formation of a carbocation from a haloalkane during the SN1 reaction of tertiary haloalkanes with sodium hydroxide.
In the previous examples, the rate determining step was one of the sequential chemical reactions leading to a product. The rate-determining step can also be the transport of reactants to interact and form the product. This case is referred to as diffusion control and, in general, occurs where the formation of product from the activated complex is very rapid and thus the supply of reactants and their interaction is rate determining.
The concept of the rate-determining step is very important to the optimization and understanding of many chemical processes such as catalysis and combustion.
In a reaction coordinate, the transition state with the highest energy is the rate-determining step of a given reaction.
References
- Zumdahl, Steven S. Chemical Principles.
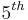 Ed. Boston, Houghton Mifflin Company: 2005, pp 727-728.
Ed. Boston, Houghton Mifflin Company: 2005, pp 727-728.
See also
|
|||||||||||||||||